The Word Entropy Is Used to Describe
Noun 1 0 A measure of the loss of information in a transmitted message. The solid wood burns and becomes ash smoke and gases all of which spread energy outwards more easily than the solid fuel.

Scalars And Vectors Physics Data Science Learning Physical Science Lessons
But its also been used to describe the approach to an imagined final state of the universe when everything reaches the same temperature.

. In this sense entropy is a measure of uncertainty or randomness. The higher the entropy meaning the more ways the system can be arranged the more the system is disordered. For example the Big Freeze theory states the Universe will eventually reach maximum entropy whereby energy reaches a state of disorder that makes it unusable for work or information storage.
The entropy is supposed to increase to a maximum then nothing will ever happen again. It is taken from the Greek word tropee which means transformation. Technically entropy from this perspective is defined as a thermodynamic property which serves as a measure of how close a system is to equilibrium that is to perfect internal disorder.
There are three important Es in the study of the thermodynamics. The entropy of an object is a measure of the amount of energy which is unavailable to do work. It usually refers to the idea that everything in the universe eventually moves from order to disorder and entropy is the measurement of that change.
Why isnt this law enough to describe all spontaneous processes. Entropy tells the amount of thermal energy which is available for useful work. In physics entropy is a quantitative measure of disorder or of the energy in a system to do work.
Responses may be used more than once. A campfire is an example of entropy. Entropy Meaning Explanation.
Entropy is defined as a state of disorder or decline into disorder. A situation of panic or disorder. Entropy is the measure of the disorder of a system.
When energy is transferred between particles by moving electrons D. Entropy is the extensive property of the system depends on the mass of the system and its unit of measurement is JK. This randomness is often collected from hardware sources either pre-existing ones such as mouse movements or specially provided randomness generators.
Thermal Engineering What is Entropy. The idea of entropy comes from a principle of thermodynamics dealing with energy. In a reversible process the total change in entropy in the system and the total change in entropy in the surroundings is zero.
Complete the following paragraph to describe energy. Describe the mathematical trick with equations used to change path functions with indefinite integrals into state functions with definite. Expert Answer 100 2 ratings Entropy is a thermodynamic property of all substances which is proportional to their degree of disorder.
From Simple English Wikipedia the free encyclopedia. When energy is transferred directly through space B. The older Ted became the faster his body fell into entropy.
Definition of Entropy a gradual fall into a state of chaos or disorder Examples of Entropy in a sentence Sue prevents her small apartment from falling into entropy by storing items in containers and on shelves. Entropy is the measure of randomness or disorder of any thermodynamic system. The German physicist Rudolph Clausius coined the term to describe the measurement of disorder and it first appeared in English in 1868.
KINETIC ENERGY or the energy of motion and POTENTIAL ENERGY or stored energy. Such a state is cold uniform and sparse with all things stopped. Apr 12 2014 Entropy is a measure of the energy dispersal in the system.
In this situation entropy is defined as the number of ways a system can be arranged. Carbon Dioxide Water- Entropy MORE Stability MORE A Messy Room vs A. When energy is transferred through matter from particle to particle C.
The ability to do work or being about a change is defined as ENERGY. In science entropy is used to determine the amount of disorder in a closed system. The meaning of ENTROPY is a measure of the unavailable energy in a closed thermodynamic system that is also usually considered to be a measure of the systems disorder that is a property of the systems state and that varies directly with any reversible change in heat in the system and inversely with the temperature of the system.
Energy equilibrium and entropy. It is an extensive property of a thermodynamic system which means its value changes depending on the amount of matter that is present. With the teacher in the hallway the classroom descended into entropy.
Write the equation for the first law of thermodynamics. What are some of the words or phrases we can use to define or describe entropy. According to Clausius the entropy was defined via the change in entropy S of a system.
We see evidence that the universe tends toward highest entropy many places in our lives. In computing entropy is the randomness collected by an operating system or application for use in cryptography or other uses that require random data. Entropy is also a measure of the number of possible arrangements the atoms in a system can have.
It occurs in two forms. An example of entropy is a stock market that is in chaos and that makes no sense and isnt predictable. In equations entropy is usually denoted by the letter S and has units of joules per kelvin JK 1 or kgm 2 s 2 K 1.
The word entropy finds its roots in the Greek entropia which means a turning toward or transformation The word was used to describe. The degree of disorder or uncertainty in. Entropy is a measure of the amount of energy that is unavailable to do work in a closed system.
Describe the difference between entropy and the second law of thermodynamics. The word entropy was first used by Rudolf Clausius. The words entropy and the second law of thermodynamics are often used interchangeably but there is a difference.
When energy is transferred through the actual movement of warmed matter. Noun 1 0 Inevitable and steady deterioration of a system or society. Entropy often comes up in theories about the ultimate fate of the Universe.

Pin By Karla Winick Ford On Ideas Entropy Thermodynamics Physics

Difference Between Enthalpy And Entropy With Definition

Difference Between Enthalpy And Entropy With Its Practical Applications In Real Life

Entropy Thermodynamics Knowino

Buamai Beyond Entropy Exhibition Guide Trend List Word Design Typography Layout Graphic Design Typography

The Idea Of Entropy Comes From A Principle Of Thermodynamics Dealing With Energy It Usually Refers To The Idea That Entropy Entropy Definition Thermodynamics

Elephant Logos Creattica Logos Retro Logos Marketing Logo

Entropy The Unseen Threat To Startups Entropy Geometry Tattoo Thermodynamics

How We Can Explain What Entropy Is About Correct Descriptions Without Including Exceptions

This Is The Victor Or Vitor Symbol From The Franco Era That Is Described In Dan Brown S Book Origin Dan Brown Books Symbols Dan Brown
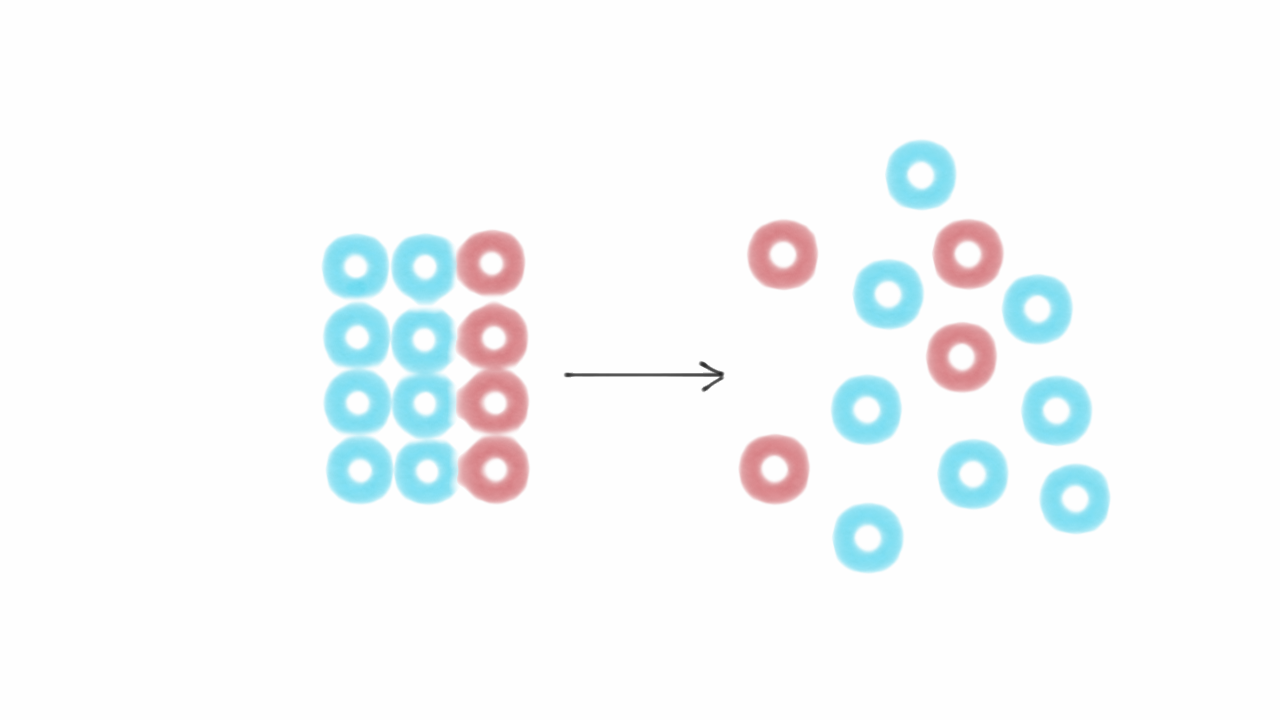
Entropy The Hidden Force That Complicates Life Farnam Street

Richard Serra Verb List Compilation Actions To Relate To Oneself 1967 1968 Verbs List Richard Serra Serra

Pin By Sarah De Silva On Chemistry High School Chemistry Chemistry Classroom Chemical Changes

Tenet The Sator Square S Cinematic And Historical Meaning Infographic Acrostic Movie Infographic




Comments
Post a Comment